The CBSE Class 10 Science Sample Paper 2026 plays a crucial role in helping students prepare effectively for the upcoming board examinations. Released by the Central Board of Secondary Education (CBSE), the sample paper is designed strictly according to the latest CBSE syllabus and exam pattern for the academic session 2025–26. It reflects the board’s focus on competency-based education, conceptual clarity, and application of scientific knowledge rather than rote memorization.
The sample paper provides students with a clear understanding of the question paper structure, including the distribution of marks, types of questions such as multiple-choice questions (MCQs), short answer, long answer, and case-based questions. Practicing the sample paper enables students to become familiar with time management, improve accuracy, and identify important chapters from Physics, Chemistry, and Biology.
Moreover, the CBSE Class 10 Science Sample Paper 2026 helps students assess their preparation level and build confidence before the final examination. Teachers and schools also use it as a benchmark to guide revision strategies and conduct pre-board exams. Overall, regular practice of the sample paper, along with proper revision, can significantly enhance performance and help students achieve better results in the Class 10 Science board examination.
CBSE Class 10 Science Sample Paper 2026
The Central Board of Secondary Education (CBSE) has officially released the Class 10 Science Sample Question Papers (SQPs) and Marking Schemes for the academic session 2025–26. These sample papers are one of the most important resources for students preparing for the board exam as they reflect the exact pattern and type of questions that can appear in the actual Science board paper.
Why Sample Papers Are Important
Sample papers help students:
- Understand the exam pattern, marking scheme, and question distribution.
- Practice time management by simulating real exam conditions.
- Identify high-weightage and frequently asked topics.
- Build confidence and reduce exam stress.
Exam Pattern of Class 10 Science 2026
According to the latest information:
- The Science exam is scheduled for February 25, 2026.
- The theory paper carries 80 marks and covers all three subjects — Physics, Chemistry, and Biology — with internal choices in some questions.
- The paper typically includes:
- Objective type questions (MCQs)
- Very short answer questions
- Short answer questions
- Long answer questions
- Case-based / Source-based questions
How to Download the Official Sample Paper
CBSE publishes the official Sample Question Papers and Marking Schemes on its academic portal. Here’s how you can check and download them:
- Visit the CBSE Academic website: cbseacademic.nic.in
- Go to the section for Sample Papers 2025-26.
- Look for Class 10 Sample Papers and select Science (Code 086).
- Download the PDF of the question paper and marking scheme.
Official CBSE Sample Papers (All Subjects, Including Science) — you can find the links here:
CBSE Sample Papers 2025-26 PDF (Class 10 & 12) — CBSE Academic Official
Additionally, you can also access sample papers hosted by educational sites that compile multiple question paper sets, for example:
- Sample Paper Set 1 (PDF) — typical format and questions.
- Multiple Set Papers (with solutions) — useful for extra practice.
Note: Always try to cross-verify with the official CBSE PDF first to ensure you follow the correct question pattern and marking scheme.
How to Use Sample Papers Effectively
To get the most out of your practice:
Practice full papers under timed conditions — this helps with pacing
Review the marking scheme — know where marks are awarded
Analyze mistakes and weak areas — rework those topics
Combine with NCERT textbook revision — most questions are based on NCERT concepts
CBSE Class 10 Science Sample Paper 2026: Key Highlights
- Designed as per the latest CBSE syllabus 2025–26
- Focus on competency-based and application-oriented questions
- Includes MCQs, short answer, long answer, and case-based questions
- Helps students understand the latest exam pattern and marking scheme
- Useful for self-assessment and revision
Preparation Tips Using CBSE Class 10 Science Sample Paper 2026
- Solve the sample paper in a 3-hour exam-like environment
- Focus on conceptual clarity instead of memorization
- Revise numericals, diagrams, and case-study questions
- Analyze mistakes and revise weak chapters
- Practice previous years’ questions along with sample papers
Chapter-wise Weightage (Expected)
- Physics: Light, Human Eye, Electricity, Magnetism
- Chemistry: Chemical Reactions, Acids & Bases, Metals & Non-metals, Carbon Compounds
- Biology: Life Processes, Control & Coordination, Heredity & Evolution, Environment
(Weightage may vary as per CBSE guidelines.)
CBSE Class 10 Science Sample Paper 2026 with Solutions
Below, we have also shared sections A and B of the sample paper questions with answers for better understanding.
Science Sample Paper 2025-2026 Section A
1. Select the group in which all organisms have the same mode of nutrition.
A. Cuscuta, yeast, legumes, leeches and tapeworm
B. Cactus, ticks, lice, leeches and cow
C. Cuscuta, ticks, lice, leeches and tapeworm
D. Cactus, grass, lice, lion and tapeworm
Answer: C. Cuscuta, ticks, lice, leeches and tapeworms; as all of these are parasites.
2 Which of the following options indicates the products formed after breakdown
of the glucose in our muscle cells when there is a lack of oxygen?
A. Ethanol + carbon dioxide + Energy
B. Lactic acid + Energy
C. Lactic acid + carbon monoxide + Energy
D. Carbon dioxide + Water + Energy
Answer: B. Lactic acid + Energy
3 Which of the following is a correct combination of function and part of the brain?
A. Posture and balance: Cerebrum
B. Salivation: Medulla in midbrain
C. Hunger: Pons in hindbrain
D. Blood pressure: Medulla in hindbrain
Answer: D. Blood pressure: Medulla in hindbrain
4 The blood glucose level in a patient was very high. It may be due to
inadequate secretion of:
A. growth hormone from pituitary gland
B. oestrogen from ovary
C. insulin from pituitary gland
D. insulin from the pancreas
Answer: D. insulin from pancreas
5 In a cross between black furred rabbit (B) and white furred rabbit (b), all offspring were found to have black fur. What can be inferred about the genetic makeup of the parent rabbits?
A. BB X bb
B. Bb X Bb
C. Bb X bb
D. bb X bb
Answer: A. BB x bb
6 Which are the correct statements related to ozone?
(i) Ozone layer helps in increasing the UV radiations reaching earth.
(ii) Ozone is a deadly poison.
(iii) Ozone layer shields the earth from UV radiations.
(iv) Ozone layer prevents UV rays which cause skin cancer.
(v) Ozone is formed with the help of Chlorofluorocarbons.
A. (i), (ii), (iii)
B. (ii), (iii), (iv)
C. (iii), (iv), (v)
D. (i), (iv), (v)
Answer: B. (ii), (iii), (iv)
7 Which of the following human activities has resulted in an increase of nonbiodegradable substances?
A. Organic farming
B. Increase in tree plantation
C. Use of plastic as packaging material
D. Composting of kitchen waste
Answer: C. Use of plastic as packaging material.
The following two questions consist of two statements – Assertion (A) and Reason (R).
Answer these questions by selecting the appropriate option given below:
A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, and R is not the correct explanation of A.
C. A is true but R is false.
D. A is false but R is true.
8. Assertion (A): Tallness of a pea plant is controlled by an enzyme.
Reason (R): The gene for that enzyme makes proteins which help the plant to be tall.
Answer: A. Both A and R are true, and R is the correct explanation of A.
9 Assertion (A): Vulture will always have the least amount of pesticides in a food chain.
Reason (R): Vulture occupies the last trophic level and it gets only 10% of energy of the previous trophic level.
Answer: D. A is false but R is true
10. Unlike animals, plants do not have any excretory products as they do not eat food. Comment upon the statement with justification.
11 Students to attempt either option A or B.
A. How many chambers are there in the heart of the following organisms?
How is mixing of oxygenated and deoxygenated blood prevented in their body?
(i) Fishes
(ii) Humans
OR
B. Explain the mechanism by which the water is transported in plants?
12. About 100 acres of forest land was declared as Natural reserve park. The following organisms were predominant in the Natural reserve park:
rabbit, frog, grass, fish, fox, water insects, zebra, peacock, snake, trees, bird, owl, insects, tiger, vulture, duck.
Create a food web comprising two separate food chains with different producers by using the above data.
13. Draw and explain how the nerve cells help in the transmission of impulses? 3
14 In a genetic experiment, plants with pure round green seeds (RRyy) were crossed with plants with wrinkled yellow seeds (rrYY).
(i) Show the gametes formed when F1 was self-pollinated.
(ii) A total of 144 seeds were produced which developed into saplings. Show the ratio in which these traits are independently inherited in these 144 sapling. 3
15 Neha consumed boiled sweet potatoes and boiled eggs for breakfast. Help her to understand some steps in the process of digestion of the food taken by her by answering the questions given below.
Attempt either subpart A or B.
A. Which of these food items is rich in protein? In which part of the alimentary canal is the digestion of this component initiated? Name the enzymes, conditions required and the glands associated with the digestion here.
OR
B. Which of these food items contains fats? How is it digested?
C. Which of these food items is rich in starch? How is its digestion initiated?
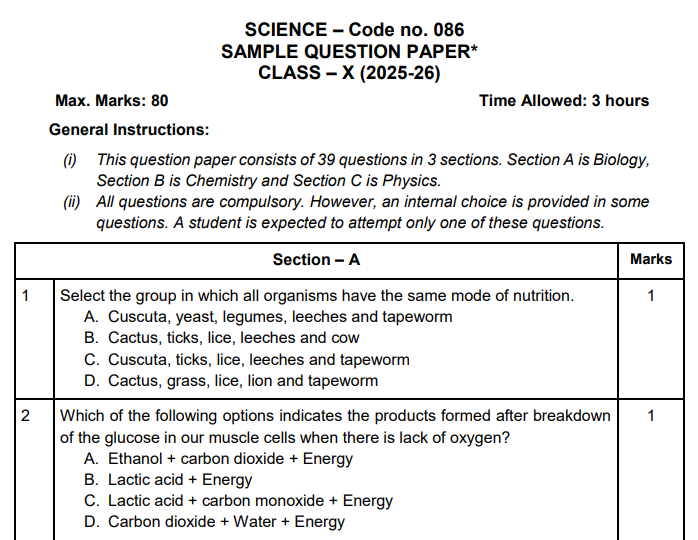
D. The figure given below represents parts of the human alimentary canal.
Which of these parts will have the maximum amount of digested food as soon as the process of digestion is completed?
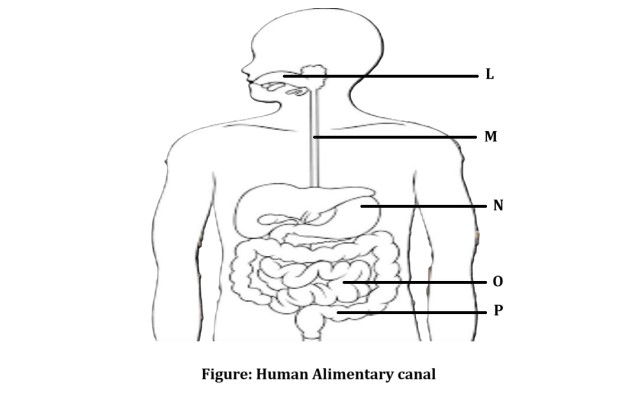
For visually impaired students
D. How will the digested food be taken up by the alimentary canal?
16 Attempt either option A or B.
A. Puneet wanted to grow banana plants.
(i) Based on your knowledge of plant reproduction, should he opt for seeds or any alternate method of reproduction? Justify your answer.
(ii) Offspring of a banana plant usually show very little variation. What causes variation and are variations good or bad? Justify.
OR
B. Annie was conducting research on the number of fruits produced by watermelon under different conditions. She grew 25 watermelon plants each in both glass house A and B. She introduced pollinators in glass house A only.
(i) What difference will she observe in the number of fruits produced in the two glass houses? Explain with reason.
(ii) List 3 changes that will occur in a flower once it gets fertilised.
Sample Paper of Science Class 10th Section B
Section – B
17. Which of the following equations represent redox reactions and what are the values for ‘p’ and ‘q’ in these equations?
Equation 1: Fe2O3(s) + 2Al(s) Al2O3 (s) + p Fe(l) + heat
Equation 2: 2C4H10(g) + 13O2(g) △ 8CO2(g)+ q H2O(g)
A. Only equation 1 is a redox reaction, p =1 and q=3
B. Both equations 1 and 2 are redox reactions, p= 2 and q=4
C. Only equation 2 is a redox reaction, p= 2 and q= 10
D. Both equations 1 and 2 are redox reactions, p= 2 and q=10
Answer: D. Both equations 1 and 2 are redox reactions, p= 2 and q=10
18 Four statements about the reactions of oxides with dilute hydrochloric acid and aqueous sodium hydroxide are listed.
I. Aluminium oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide.
II. Calcium oxide reacts with dilute hydrochloric acid and aqueous sodium hydroxide.
III. Zinc oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide.
IV. Sulphur dioxide does not react with either dilute hydrochloric acid or aqueous sodium hydroxide.
Which statements are correct?
A. I and II
B. I and III
C. II and IV
D. III and IV
Answer: B. (I) and (III)
19. An iron nail is added to each of the two test tubes ‘P’ and ‘Q’ containing aqueous copper (II) sulphate, and aqueous silver nitrate, respectively. Which of the following observations is correct?
A. In test tube ‘P’ iron nail is coated with a blue coating and in test tube ‘Q’ there is no reaction.
B. Iron nail is coated with a brown coating in test tube ‘P’ and silver coating in test tube ‘Q’.
C. There is no reaction in either of the test tubes ‘P’ or ‘Q’.
D. There is no reaction in test tube ‘P’ but a silver coating on iron nail is seen in test tube ‘Q’.
Answer: B. Iron nail is coated with a brown coating in test tube ‘P’ and silver coating in test tube ‘Q’.
20. Methyl orange is added to dilute hydrochloric acid and to aqueous sodium hydroxide. What is the colour of the methyl orange in each solution?
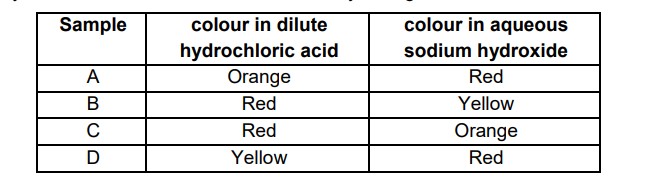
Answer: B. Red Yellow
21 Which of the following substances when dissolved in equal volume of water, will have the highest pH value?
A. Sulphuric acid
B. Acetic acid
C. Magnesium hydroxide
D. Sodium hydroxide
Answer:D. Sodium hydroxide
22 When excess of carbon dioxide is passed through lime water, the milkiness disappears because
A. water soluble calcium carbonate converts to water soluble calcium
bicarbonate.
B. insoluble calcium carbonate converts to water soluble calcium
bicarbonate.
C. Water-soluble calcium carbonate converts to insoluble calcium
bicarbonate.
D. insoluble calcium carbonate converts to insoluble calcium bicarbonate.
Answer:B. Insoluble calcium carbonate converts to water-soluble calcium bicarbonate.
23 In the reaction of aqueous solution of barium chloride with aqueous solution of sodium sulphate, the aqueous solution formed will be:
A. BaCl2
B. BaSO4
C. Na2SO4
D. NaCl
Answer: D. NaCl
[extra_info id=51]
The following question consists of two statements – Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below:
A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, and R is not the correct explanation of A.
C. A is true but R is false.
D. A is false but R is true.
24 Assertion (A): C4H8, C4H6 and C4H10 are members of the same homologous series
Reason (R): C4H8, C4H6, C3H4, C3H6, C2H4, C2H2 are unsaturated hydrocarbon
Answer: D. A is false, but R is true
25. The following activity is set up in the science lab by the teacher.
He clamped an aluminium wire on a stand and fixed a pin to the free end of the wire using wax. Then he heated the wire with a burner from the end where the wire is clamped. Students observed the pin fall off.
A. If the teacher replaces aluminium wire by silver wire, will the students’ observation change? Justify your answer.
B. Will the aluminium wire melt? Give a reason for your answer. 2
26 Attempt either option A or B.
A. An element ‘X’ is stored in kerosene and cannot be extracted from its ore using a reducing agent. ‘X’ forms an ionic compound on reaction with chlorine.
(i) Can we store ‘X’ in water? Give a reason to support your answer.
(ii) Identify element ‘X’. Name the process used and write the equation for the extraction of ‘X’ from its ore.
OR
B. The domes of many buildings in Europe are made of copper. These domes now appear greenish in colour.
(i) Why do the domes appear greenish though copper is orange-red in colour?
(ii) In your opinion, should the copper domes be replaced by iron domes to overcome the problem of change of colour of copper domes?
(iii) Domes used to be made from thin sheets of metals. Why did the ancient architects use copper to make domes?
CBSE Class 10 Science Sample Paper 2026 Question Paper PDF
Download Class 10 Science Sample Paper by Clicking on the link below:
Class 10 Science Sample Paper-SQP
Related Sample Paper Links:
FAQs on CBSE Class 10 Science Sample Paper 2026
Q1. Is the CBSE sample paper enough for board preparation?
It is essential but should be combined with NCERT textbooks and PYQs.
Q2. Are case-based questions compulsory?
Yes, CBSE emphasizes competency-based and case-study questions.
Q3. From where should students download the official sample paper?
From the official CBSE academic website.
Q4. Does the sample paper include internal choice?
Yes, internal choices are provided as per CBSE norms.